A battery’s state of charge is a percentage that reflects the amount of energy it stores relative to its capacity. It provides information on how much power it has left within it and the approximate time it can deliver a sustainable power.
Understanding the battery’s state is essential to a battery’s upkeep or maintenance because it can be a basis that tells if the cell is bad or not.
There are several ways to determine the state of charge of a battery, but only one stands out when it comes to accuracy. It is the method of determining the specific gravity of the cell, which uses a hydrometer.
What is the hydrometer reading for a fully charged battery? A fully charged battery should have a hydrometer reading of its electrolyte’s specific gravity within the range of 1.265 to 1.330. That is depending on the kind of battery and the manufacturer of the battery.
Although Battery Council International or BCI specified that the specific gravity of a fully charged cell is 1.265, some manufacturers prefer 1.280 or more. The specific gravity of a fully charged battery also varies on the battery application, which also varies according to the expected cell performance.
Deep cycle batteries have a specific gravity between 1.270 and 1.305. Other factors affect the value of specific gravity, like the acidity of the electrolyte and ambient temperature.
This is why the ideal time to carry out a specific gravity test when the battery is fully charged and rested about three to five hours.
This gives the electrolyte within the cell enough time to stabilize, which will lead to a more accurate reading. Again, if a battery is fully charged, your hydrometer should read a specific gravity of 1.265 or more.
For flooded lead-acid batteries, the most accurate way of telling its state of charge is using a hydrometer and measuring the electrolyte’s specific gravity. With the specific gravity reading on hand, it will then be looked on a chart that will have the information about its state of charge equivalent.
How Batteries Work in Relation to the Specific Gravity
When a lead-acid battery is in the charging state, its operation is to receive charge that it will store on its plates within. At this state, the electrolyte also accumulates more sulfuric acid that makes its acidity more potent.
Due to this, the acid’s density within the electrolyte becomes denser than water, increasing its specific gravity.
On that note, the higher the battery’s specific gravity, the better the state of charge it will have. When checking a battery’s specific gravity, the process will require you to get a reading from all of the battery cells.
This means that you will have several interpretations, and with that said, you will be able to see the state of charge of each cell. And then, you will be taking the readings and compare it to a chart to see the state of charge.
The Standard State of Charge and Specific Gravity Chart from BCI
As I mentioned earlier, after getting the specific gravity reading of the battery, you will have to check that reading on a chart that will tell the estimated state of charge.
Here’s a table with the standard values from the Battery Council International. This chart shows the standard values for two-volt, six-volt, eight-volt, and twelve-volt batteries.
The readings on the table are based on 78°F temperature, where the cells are rested for twenty-four hours.
The table below shows the open-cell voltage or rest voltage of each cell and the equivalent specific gravity as well as the state of charge.
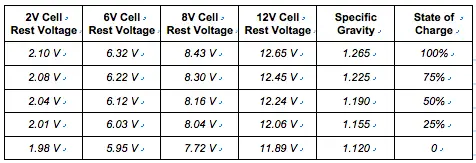
Credit for Battery Council International
You have to remember that there are several factors that may affect the specific gravity reading on a battery.
And one of these factors is temperature, both the ambient temperature and the temperature of the electrolyte can change the readings. That is why it is recommended that the battery is rested before carrying out the test.
How the Electrolyte Temperature Affect the Specific Gravity Readings
The electrolyte temperature has a direct relationship with the specific gravity of the battery, leading to discrepancies in the reading on hydrometers. When the electrolyte gets colder, it becomes denser, which also raises the specific gravity.
On the other hand, when it gets hotter, the electrolyte is lighter, which decreases the specific gravity. To get a better picture of these changes, here’s a table to refer to.

Other Factors that Can Affect the Specific Gravity Readings on a Battery
Apart from the state of charge of the battery, voltage, and temperature, several factors can also lead to inaccurate specific gravity readings.
Stratification is one of these other factors that can lead to inaccurate hydrometer readings. This is a condition where the battery electrolyte has a layered concentration, which limits its capability to be efficient.
This condition usually leads to sulfation, which is one of the leading causes of a battery’s death because it prevents it from storing charge.
Higher acid potency on the electrolyte of the cell also raises the rest voltage of the battery, which can alter the hydrometer’s reading.
However, when carrying out a specific gravity that you have to keep in mind, the general rule is to let the battery rest for at least four hours to twenty-four hours before testing.
Proper Way of Using a Hydrometer to Get Specific Gravity
In case you need to carry out a specific gravity test on your flooded lead-acid battery for maintenance purposes and you don’t know-how.
Here’s a guide on how to use a hydrometer and a step by step guide of the entire process. Let me walk you through each step and help you successfully carry out the operation.
1. Equip the Appropriate Safety Gear
Since you are going to deal or work with a wet cell battery with hazardous elements involved, the first thing you have to do is to make sure that you are protected by wearing safety goggles and a pair of rubber gloves.
You also have to procure a reliable hydrometer to be sure that you are going through each step smoothly.
2. Prepare the Battery for the Specific Gravity Testing
The next thing you have to do is prepare the battery by placing it on a flat surface and removing each cell’s lead. Place all the lids aside because you will be replacing them once you are done with the process.
3. Warm the Hydrometer Up and Observe Electrolyte Coloration
Start with one cell of the battery and then draw some electrolytes to the instrument. But do it several times to warm the device up and adjust with the electrolyte’s temperature.
After that, examine the color of the electrolyte, if it has a brown or gray coloration, that means the battery is dying.
4. Start Getting Readings of the Specific Gravity
Draw electrolyte samples to the instrument with a full dosage until the float of the instrument is floating freely. Hold the instrument at eye level to see where the battery fluid meets the scale of the device. Make sure to take note of the reading.
5. Correct the Reading With the Temperature Correction
To get a more accurate reading, make sure to add or subtract 0.004 on the reading for every 10° F or 6° C the electrolyte temperature is above the normal basis which is 80° F or 27° C.
Then from there, note the reading for the first cell then continue with the others, doing the same. Once you have all of the readings for each cell noted and corrected, you can compare them with the table given earlier.
Based on the table and the readings you got, you can tell the state of charge of the battery and see if something is wrong with it or not. Just make sure that the battery is fully rested before carrying out the process.
Final Thoughts
That is how you use a hydrometer and see the state of charge of a flooded lead-acid battery. All of the information disclosed above will also allow you to learn everything about the specific gravity, state of charge, and voltage.
Just remember, a fully charged battery should have a hydrometer reading of 1.265 specific gravity or more.